
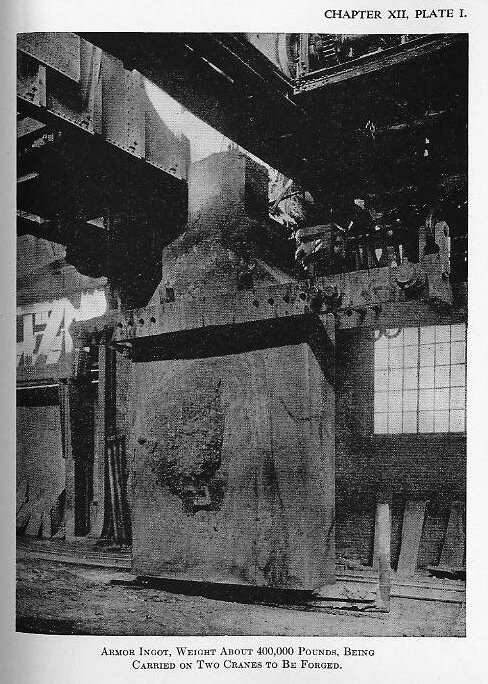
 (2) The ingot, while still hot, is stripped from the mould, cleaned, and prepared for forging (Plate I).
(2) The ingot, while still hot, is stripped from the mould, cleaned, and prepared for forging (Plate I).
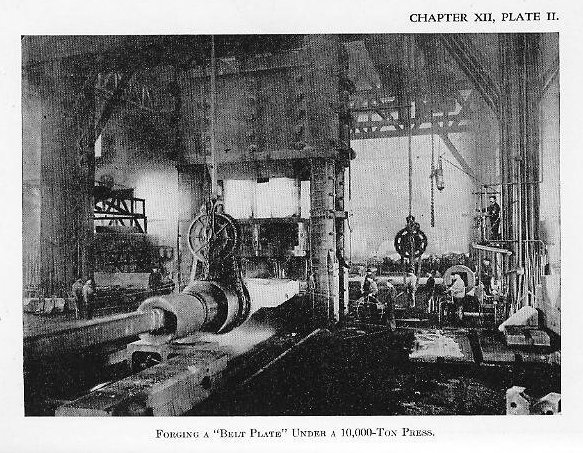 {3) The ingot is then reheated and forged under a hydraulic press to within about 15 per cent of the final thickness. The forging reduces the ingot to about one-third of its previous thickness. The segregation of impurities in the central upper portion is discarded by cutting off (Plate II).
{3) The ingot is then reheated and forged under a hydraulic press to within about 15 per cent of the final thickness. The forging reduces the ingot to about one-third of its previous thickness. The segregation of impurities in the central upper portion is discarded by cutting off (Plate II).
 (4) The forging is annealed to produce a partially fibrous condition of the microstructure to prevent cracking in cooling and to eliminate the strains due to forging.
(5) It is then super-carburized (Plate III). The time required for this step varies with the size of the forging; large forgings take from 10 to 14 days.
(4) The forging is annealed to produce a partially fibrous condition of the microstructure to prevent cracking in cooling and to eliminate the strains due to forging.
(5) It is then super-carburized (Plate III). The time required for this step varies with the size of the forging; large forgings take from 10 to 14 days.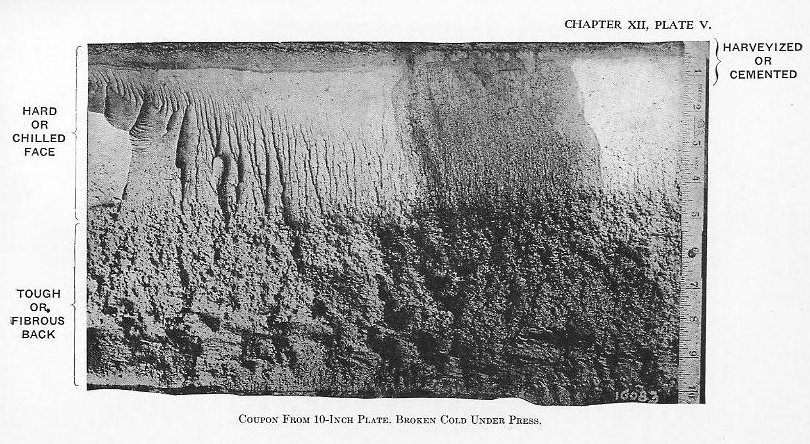 (7) Several heat treatments may follow to develop the proper physical properties in the fiber (Plate V).
(7) Several heat treatments may follow to develop the proper physical properties in the fiber (Plate V). 10) The front face is then heated above the critical temperature, depending upon the depth of chill desired, and hardened by oil or water spraying (Plate IV).
10) The front face is then heated above the critical temperature, depending upon the depth of chill desired, and hardened by oil or water spraying (Plate IV).